antimicrbial food-drug indication
Same asabove - Prontosan Wound Gel X is indicated for the cuts. Establishing durations of use for these drugs and updating product dosage regiments supports FDAs antimicrobial stewardship efforts.
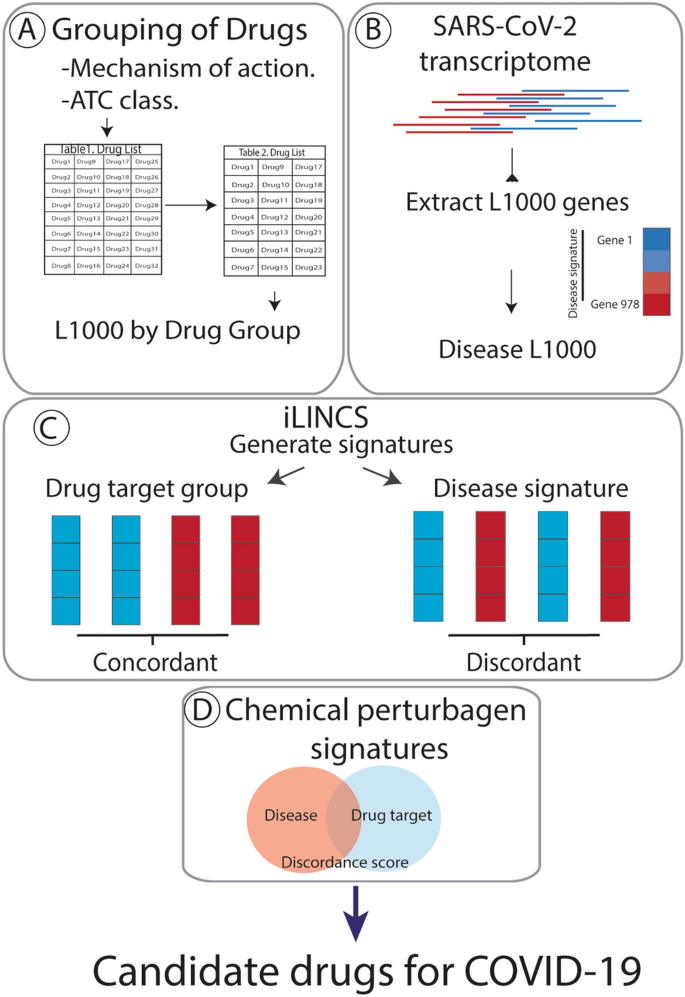
Identification Of Candidate Repurposable Drugs To Combat Covid 19 Using A Signature Based Approach Scientific Reports
Importer in relation to an imported article includes any person who whether as owner consignee agent or broker is in.
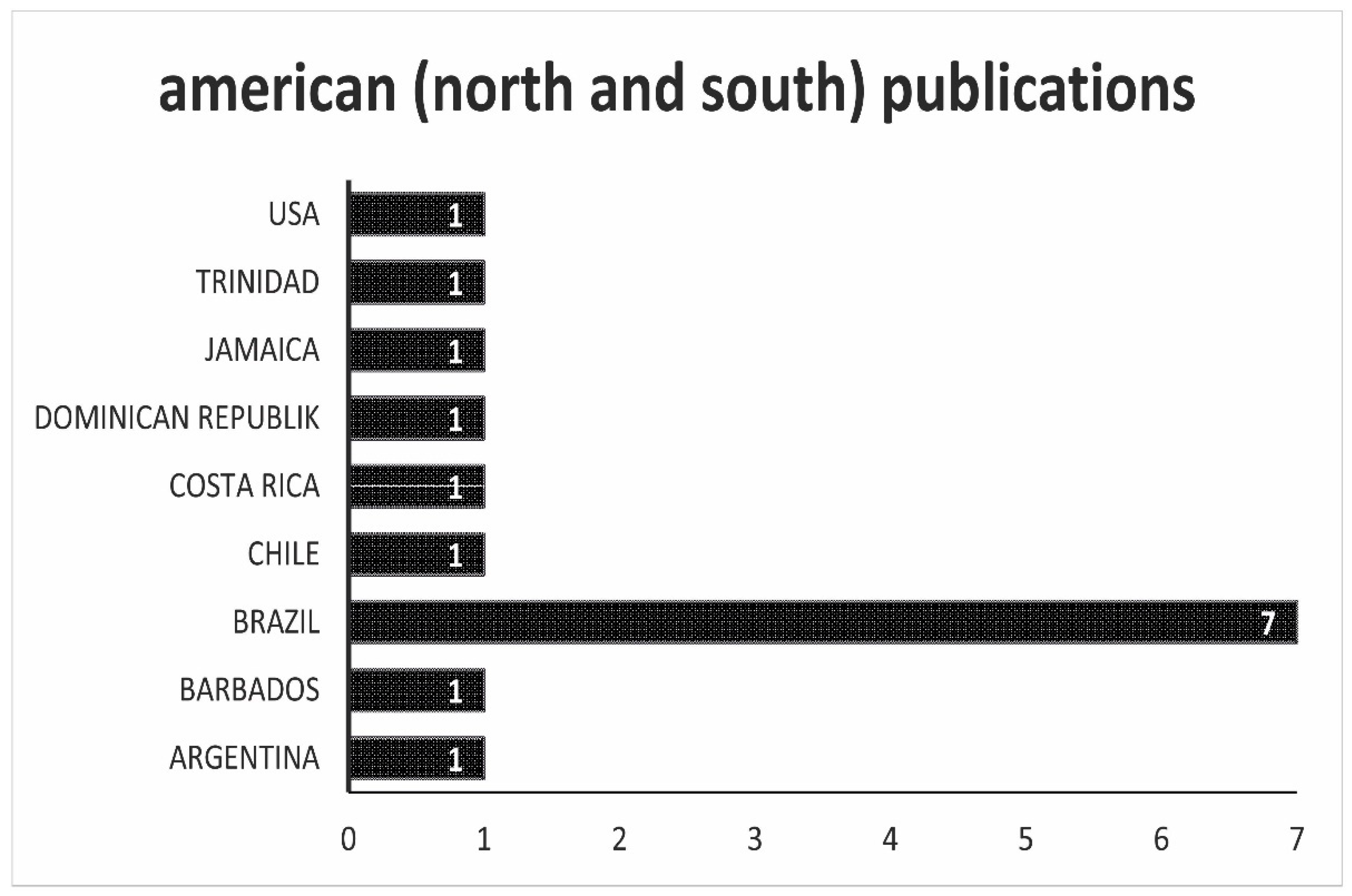
. Producing species and they are regulated as food. Antimicrobial Drug-Food Interactions Compiled April 2008 Indinavir Food Food may decrease bioavailability of indinavir Take on an empty stomach at least 1 hour before or 2 hours after a meal. Drugs Food Drug-Food Interaction.
However this link between agricultural antibiotic use and antibiotic resistance has remained contested by. Cloudy urine Fever Pain. Antimicrobial Drugs Approved for Use in Food-Producing Animals Actively Marketed in 2009 Domestic Sales and Distribution Data Reported by Medical Importance Route of Adminstration and Drug Class.
What is an antibiotic indication. 510k Number if known K160223 Device Name VentriClear Ventricular Drainage Catheter Set with Cook Spectrum Antibiotic Impregnation VentriClear II Ventricular Drainage Catheter Indications for Use. New antimicrobial agents approved by the US.
Background Section 105 of the Animal Drug User Fee Amendments of 2008 ADUFA PL. FDA has published a list of approved medically important antimicrobial drugs administered in the feed of food-producing animals that lack a defined duration of use. Food and Drug Administration Indications for Use Form Approved.
Interferes with the effectiveness and safety of warfarin therapy. It is an approach to maximize therapeutic efficacy and minimize selection of resistant microorganisms. Medically reviewed by Leigh Ann Anderson PharmDLast updated on Sep 17 2021.
Food and Drug Administration in 1997 and New Indications for Previously Approved Agents on page 987. Raise serum albumin levels decrease in international normalized ratio INR Vegetables containing vitamin k. Food and Drug Administration in 1999 and.
Drink water skim or non-fat milk juice coffee or tea when taking the medicine. An antibiotic indication is the reason for antibiotic use either an infection being treated or prophylaxis against an infection. We review historical availability and regulation and recent indications of antimicrobial drugs for food animals in the USA.
We summarize the timeline of introduction of individual antimicrobial drug classes from the 1930s to present history of regulation of antimicrobial drugs from the 1930s to present and indications of antimicrobial drugs in 1996-2014 for food. Because antimicrobial consumption in food-producing animals contributes to the problem policies restricting the inappropriate or unnecessary agricultural use of antimicrobial drugs are important. When To Use When NOT To Use Top 10 Infections Treated Top 10 Generic Drugs Top 10 Brand Drugs Antibiotic Class Types OTC Options More Resources.
Page 3 I. Judicious use of therapeutic antimicrobials is an integral part of good veterinary practice. Indications or use.
Food and Drug Administrations Guidance for Industry 2131a policy designed to ensure the judicious. 3001 5 food includes any article manufactured sold or representedfor use as food or drink for man chewing gum and any ingre dient that maybe mixed withfood for anypurposewhatever. Antimicrobial resistance is a public health threat.
Indications Same as for management of minoruse Atteris Antimicrobial Barrier Film Dressing is intended for application to minor wounds and damaged skin as a liquid film forming barrier which creates a waterproof film dressing protecting the wound or damaged skin. Food and Drugs Chap. If you need to take the medicine with food eat a small.
These recommendations for sponsors of approved medically important antimicrobial drugs administered in feed or water to food-producing animals include. 10903 New Hampshire Avenue Silver Spring MD 20993 Ph. Antimicrobial drugs approved for use in or on the feed or drinking water of food-producing animals worked with FDA to voluntarily withdraw approval of indications that were not considered.
We systematically reviewed published literature for evidence of a relationship between antimicrobial use in agricultural animals and drug-resistant meat or dairy-borne non-typhoidal salmonellosis in humans. Lamisil emulsion cream Terbinafine hydrochloride. Controversy continues concerning antimicrobial use in food animals and its relationship to drug-resistant infections in humans.
1-888-INFO-FDA 1-888-463-6332 Contact FDA. Judicious Animal Antibiotic Use Requires Drug Label Refinements Analysis shows more than 1 in 3 labels will not fully meet judicious use standards after implementation of FDA policy A brief from Oct 2016 Overview The US. Expansion of labeling to include patients with onychomycosis of fingernail or toenail even when associated with nondermatophytes.
The Antimicrobial Drug Advisory Committee of the Food and Drug Administration Center for Drug Evaluation and Research met on April 26 2019 at the. For prevention of Pneumocystis carinii. 1 Removing production indications eg increased rate of weight gain and improved feed efficiency and 2 incorporating veterinary oversight for the remaining therapeutic indications.
This corrects the article New Antimicrobial Agents Approved by the US. January 31 2017 See PRA Statement below. Every antibiotic needs to come with an indication for use like radiology and PRN orders need indications.
3509 amended section 512 of the Federal Food Drug and Cosmetic Act the Act. Youve most likely taken an antibiotic or anti-infective at least once in your. These are also the species for which the drugs on the prohibited list could not be used in an extra-label manner.
To evaluate the effect of food on bioavailability clarithromycin and 14-hydroxyclarithromycin active metabolite pharmacokinetics were assessed in 26 healthy adult volunteers after ingestion of a single oral 500-mg dose of clarithromycin in a fasting state 2 hours before breakfast after an overnight fast and a nonfasting state 05 hours after the start of breakfast. Many species of animals are considered to be food-producing animals and human food safety review is required prior to approval of a new animal drug for use in that species.

Waves Of Attention Patterns And Themes Of International Antimicrobial Resistance Reports 1945 2020 Bmj Global Health

Short History Of Regulations And Approved Indications Of Antimicrobial Drugs For Food Animals In The Usa Volkova 2017 Journal Of Veterinary Pharmacology And Therapeutics Wiley Online Library

Short History Of Regulations And Approved Indications Of Antimicrobial Drugs For Food Animals In The Usa Volkova 2017 Journal Of Veterinary Pharmacology And Therapeutics Wiley Online Library

Antibiotics Free Full Text Antimicrobial Residues In Food From Animal Origin A Review Of The Literature Focusing On Products Collected In Stores And Markets Worldwide Html
Antibiotics From Nature Traditional Medicine As A Source Of New Solutions For Combating Antimicrobial Resistance Amr Control
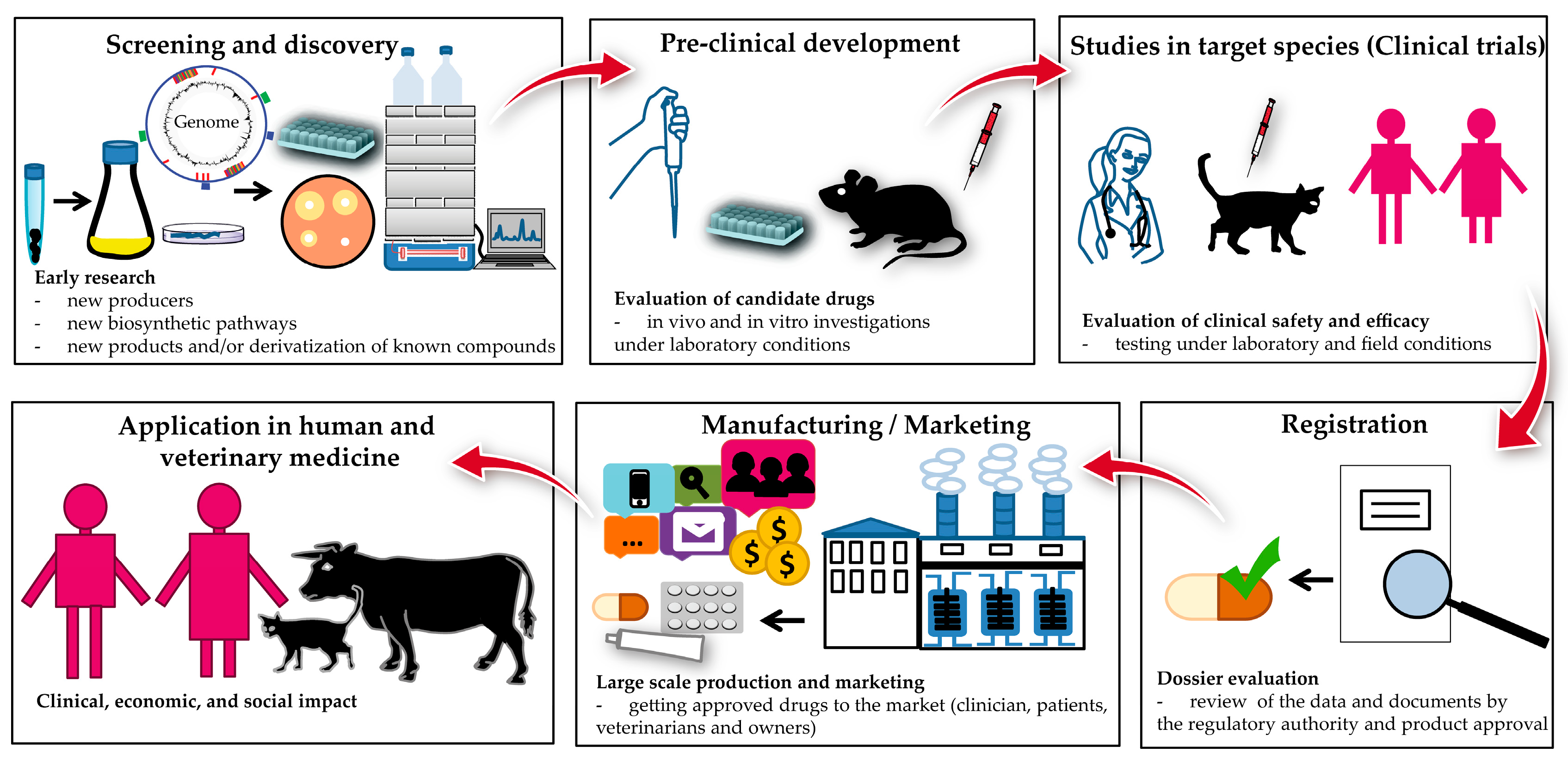
Antibiotics Free Full Text Actinomycete Derived Polyketides As A Source Of Antibiotics And Lead Structures For The Development Of New Antimicrobial Drugs Html
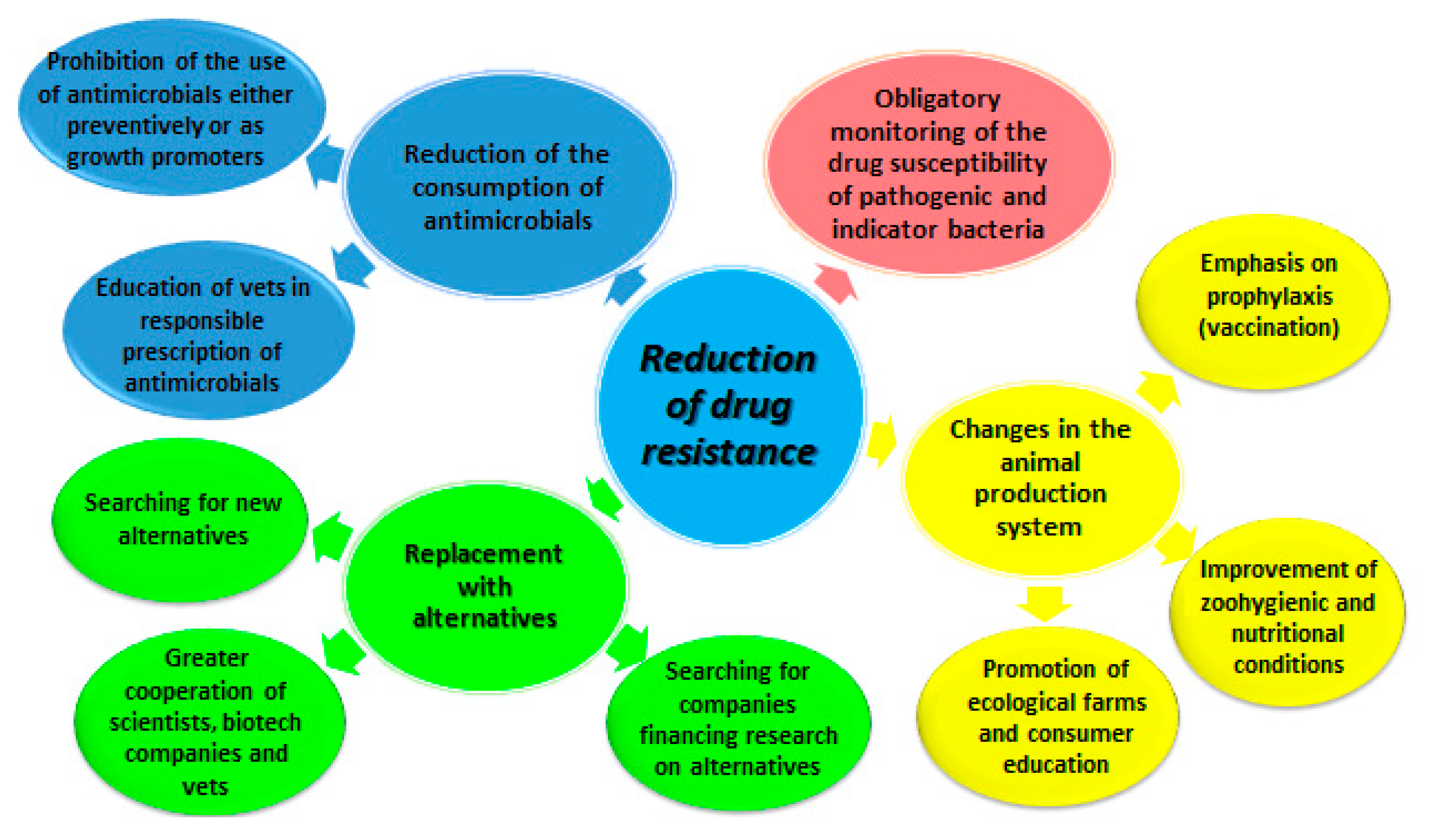
Antibiotics Free Full Text Last Call For Replacement Of Antimicrobials In Animal Production Modern Challenges Opportunities And Potential Solutions Html
0 Response to "antimicrbial food-drug indication"
Post a Comment